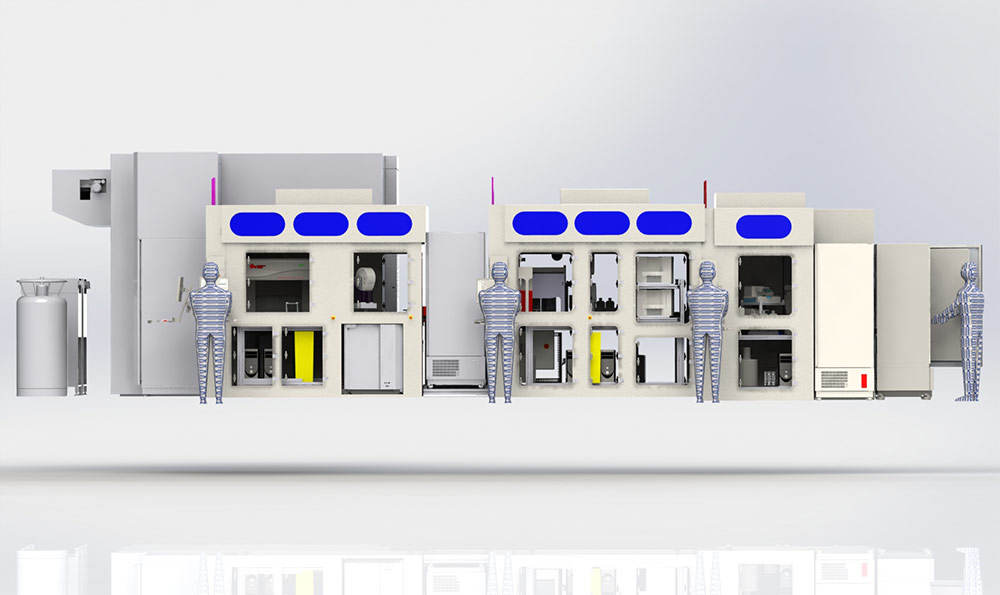
PAA delivered a VRSS Antibody discovery platform to UCB. The multi-workcell platform screens β-lymphocytes to find the ones that produce the right antibodies for use in biopharmaceuticals.
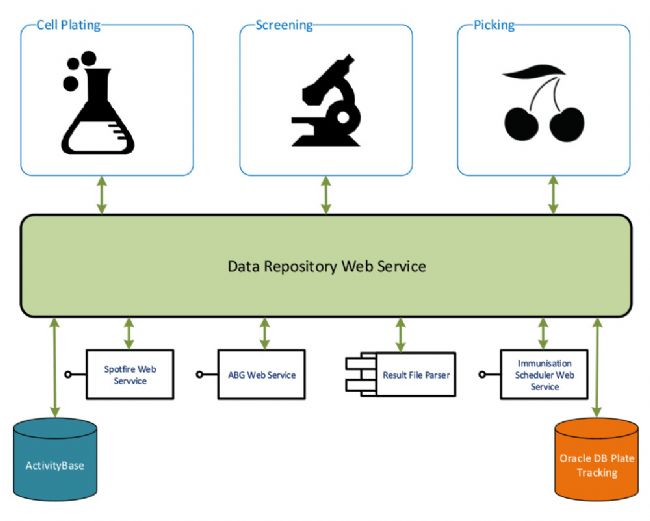
The platform is based on PAA’s S-CEL™ technology. The modular platform sets up cultures, screens them and then selects “hits”, which are then moved to a -80˚C store. The scientists at UCB interact with the system using the Harmony™ touch-screen user interface software. Each module is controlled by its own incident of PAA’s Overlord™ scheduler. Overlord coordinates the entire sequence of operation and completes the integration with the UCB data repositories.
The platform has the capacity to screen over a billion β-lymphocytes, a substantial increase in throughput over the previous methods of screening.
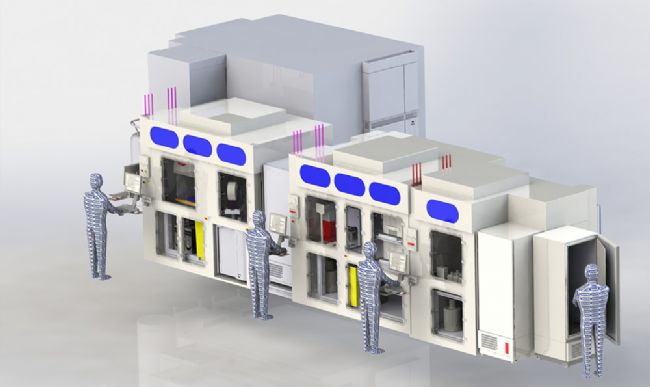
The automated system itself is built around three integrated PAA S-CEL™ class II type containment workcells, with made-to-measure dimensions. Adhering to the relevant ISO standards, the laminar flow setup maintains a sterile environment to protect against contamination risk, and the workcells manage separate processes as follows:
Cell culture – In the filling workcell, B-cells collected from samples immunised with the target of interest are seeded into barcoded 96-well plates, with a typical experiment involving more than 500 cell plates. Over seven days of incubation, B-cell clonal expansion and differentiation into antibody-secreting cells occurs, and antibody is secreted into the supernatant. Cell plates are then placed on a conveyor link to the Screening Workcell.
Antibody Screening – In a second workcell dedicated to antibody screening, secreted antibodies on the cell plates are assayed for binding and functional activity. The supernatant is first screened for antigen-specific antibody, with 10 µl of supernatant removed from the cell culture plate into a 384-well assay plate. A homogenous fluorescence binding assay measures specific binding through reading fluorescence intensity, with an intensity of 10% or less in a single well indicating that the signal derives from only one B-cell clone. Sample identification data from the original cell plates is tracked through the system and linked with the assay plate. Following data acquisition with a high content screening instrument, the PAA data handling module will take the CSV data files and through ActivityBase, analyse the data against an operator-defined threshold. From this, a plate map of positive hits is produced for the user to review and validate, forming a pick list for the Hit Picking Workcell.
Hitpicking – A third workcell is optimised for hitpicking, and supernatant from the positive hits is transferred from the cell plates to a single consolidated master plate. Further analysis with complex functional and biochemical assays enables UCB to characterise the antibody in more detail. In parallel, cell plates containing the positive hits are stored at -80°C. Full sample traceability through the system is provided, and data linking the master plate to the cell plates is stored within a custom company database, hosted within both Oracle and IDBS ActivityBase software. Once hits that match a panel of requirements are identified by subsequent screening, the B-cell clones of interest are isolated for cloning and further development using UCB’s novel fluorescence foci method
VRSS Antibody Discovery Platform – Design Phase

VRSS Antibody Platform – Data Management
Increasing capacity and quality with a fully automated system
Enabling the screening of more than one billion B-cells in a single campaign, automating UCB’s antibody discovery programme with fully integrated data handling is driving the discovery of rare antibodies across a diverse array of targets and species. UCB is now able to mine the entire immune repertoire for high-quality antibodies in a shorter time, helping the company deliver efficacious therapies faster. This is achieved through a host of advantages:
Increased capacity to 400 plates per day, allowing UCB to run more projects in parallel
Allows antibody selection based on highly stringent criteria, generating high-quality hits
Exact, repeatable processing yields reproducible data
Completely sterile operation
Intuitive operation is ideal for users with minimal training
Scheduling provides tight control of incubation times, standardising assay conditions for reliable results
System has flexibility for multiple plate batches, screening multiple cell lines against multiple compounds
Provides a full inventory and electronic audit trail for the entire process
bio-equip.cn