INTENDED USE
The FOB Rapid Test Device (Feces) is a rapid visual immunoassay for the qualitative presumptive detection of human hemoglobin in human fecal specimens. This kit is intended to be used as an aid in the diagnosis of lower gastrointestinal (g.i.) pathologies.
INTRODUCTION
Colorectal cancer is one of the most commonly diagnosed cancers and a leading cause of cancer death in the United States. Screening for colorectal cancer probably increases the cancer detection at an early stage, therefore reduces the mortality.
Earlier commercially available FOB tests utilized the guaiac test, which requires special dietary restriction to minimize false positive and false negative results. The FOB Rapid Test Device (Feces) are especially designed to detect human hemoglobin in fecal samples using Immunochemical methods, which improved specificity for the detection of lower gastrointestinal. disorders, including colorectal cancers and adenomas.
KIT COMPONENTS
|
Individually packed test strips
|
Each strip contains colored conjugates and reactive reagents pre-spreaded at the corresponding regions.
|
|
Specimens collection cards
|
For specimens collection use.
|
|
Specimens dilution tube with buffer
|
Each contains 2 ml of 0.1 M Phosphate buffered saline (PBS) and 0.02% sodium azide.
|
|
Package insert
|
For operation instruction.
|
PROCEDURE
Bring tests, specimens, buffer and/or controls to room temperature (15-30°C) before use.
1.Specimen collection and pre-treatment:
1) Use the specimens collection cards provided in the kit for specimens collection. Follow the operation procedure written on it for instructions. Other clean dry containers could also be used for the same purpose. Best results will be obtained if the assay is performed within 6 hours after collection.
2) Unscrew and remove the dilution tube applicator. Be careful not to spill or spatter solution from the tube. Collect specimens by inserting the applicator stick into at least 3 different sites of the feces.
3) Place the applicator back into the tube and screw the cap tightly. Be careful not to break the tip of the dilution tube.
4) Shake the specimen collection tube vigorously to mix the specimen and the extraction buffer. Specimens prepared in the specimen collection tube may be stored for 6 months at -20°C if not tested within 1 hour after preparation.
2. Testing
1) Remove the test from its sealed pouch, and place it on a clean, level surface. Label the test with patient or control identification. To obtain a best result, the assay should be performed within one hour.
2) Using a piece of tissue paper, break the tip of the dilution tube. Hold the tube vertically and dispense 3 drops of solution into the specimen well (S) of the test device.
Avoid trapping air bubbles in the specimen well (S), and do not drop any solution in observation window.
As the test begins to work, you will see color move across the membrane.
3. Wait for the colored band(s) to appear. The result should be read at 5 minutes. Do not interpret the result after 10 minutes
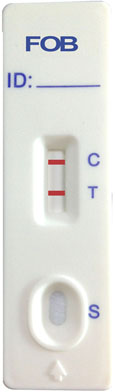
INTERPRETATION OF RESULTS

bio-equip.cn