INTENDED USE
The Strep A Rapid Test Device (Throat Swab) is a rapid chromatographic immunoassay for the qualitative detection of Strep A antigen from throat swab specimens to aid in the diagnosis of Group A Streptococcal infection.
INTRODUCTION
Streptococcus pyogenes is non-motile gram-positive cocci, which contains the Lancefield group A antigen that can cause serious infections such as pharyngitis, respiratory infection, impetigo, endocarditis, meningitis, puerperal sepsis, and arthritis.1 Left untreated, these infections can lead to serious complications, including rheumatic fever and peritonsillar abscess.2 Traditional identification procedures for Group A Streptococci infection involve the isolation and identification of viable organisms using techniques that require 24 to 48 hours or longer.3,4
The Strep A Rapid Test Device (Throat Swab) is a rapid test to qualitatively detect the presence of Strep A antigen in throat swab specimens, providing results within 5 minutes. The test utilizes antibodies specific for whole cell Lancefield Group A Streptococcus to selectively detect Strep A antigen in a throat swab specimen.
KIT COMPONENTS
- Test devices
- Sterile swabs
- Strep A Reagent A
|
(1M Sodium Nitrite)
|
(Non-viable Strep A; 0.09% NaN3)
|
- Workstation
- Test tubes
- Strep A Reagent B
|
(0.4M Acetic Acid)
|
(Non-viable Strep C; 0.09% NaN3)
|
- Dropper tips
- Package insert
|
|
|
PROCEDURE
Allow the test device, reagents, throat swab specimen, and/or controls to reach room temperature (15-30°C) prior to testing.
1.Remove the test device from the sealed foil pouch and use it as soon as possible. Best results will be obtained if the test is performed immediately after opening the foil pouch.
2.Hold the Reagent A bottle vertically and add 4 full drops (approximately 240 mL) of Reagent A to an extraction test tube. Reagent A is red in color. Hold the Reagent B bottle vertically and add 4 full drops (approximately 160 mL) to the tube. Reagent B is colorless. Mix the solution by gently swirling the extraction test tube. The addition of Reagent B to Reagent A changes the color of the solution from red to yellow. See illustration 1.
3.Immediately add the throat swab to the extraction test tube of yellow solution. Agitate the swab 10 times in the tube. Leave the swab in the tube for 1 minute. Then press the swab against the side of the tube and squeeze the bottom of the tube as the swab is withdrawn. Discard the swab. See illustration 2.
4.Fit the dropper tip on top of the extraction test tube. Place the test device on a clean and level surface. Add 3 full drops of solution (approx. 100 µL) to the specimen well (S) and then start the timer. See illustration 3.
5.Wait for the colored line(s) to appear. Read the result at 5 minutes. Do not read the result after 10 minutes.
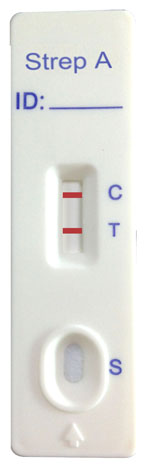
INTERPRETATION OF ASSAY RESULT

PERFORMANCE CHARACTERISTICS
Table: Strep A Rapid Test vs. PCR Test
|
Method
|
Culture
|
Total Results
|
|
Strep A
Rapid Test
|
Results
|
Positive
|
Negative
|
|
Positive
|
102
|
7
|
109
|
|
Negative
|
6
|
377
|
383
|
|
Total Results
|
108
|
384
|
492
|
Relative Sensitivity: 94% (88%-98%)* Relative Specificity: 98% (96%-99%)*
Accuracy: 97% (96%-98%)* * 95% Confidence Intervals
bio-equip.cn