[PACKAGE QUANTITY]
25 tests/kit
[APPLICATION]
The HCV Rapid Test Device (Serum/Plasma) is a rapid visual immunoassay for the qualitative presumptive detection of antibodies to HCV in human whole blood, serum, or plasma specimens. This kit is intended to be used as an aid in the diagnosis of HCV infection.
[TEST PRINCIPLE]
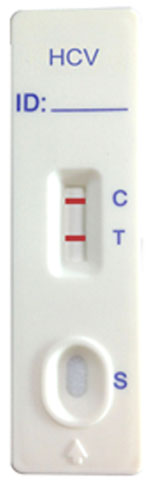
The HCV Rapid Test Device (Whole Blood/Serum/Plasma) has been designed to detect antibodies to HCV through visual interpretation of color development in the internal strip. The membrane was immobilized with protein A on the test region. During the test, the specimen is allowed to react with colored recombinant HCV antigens colloidal gold conjugates, which were precoated on the sample pad of the test. The mixture then moves on the membrane by a capillary action, and interact with reagents on the membrane. If there were enough HCV antibodies in specimens, a colored band will from at the test region of the membrane. Presence of this colored band indicates a positive result, while its absence indicates a negative result. Appearance of a colored band at the control region serves as a procedural control. This indicates that proper volume of specimen has been added and membrane wicking has occurred.
[MAIN COMPONENTS]
|
Test cassette
|
25pcs
|
|
|
Package insert
|
1pcs
|
|
|
Buffer
|
1 bottle
|
|
bio-equip.cn