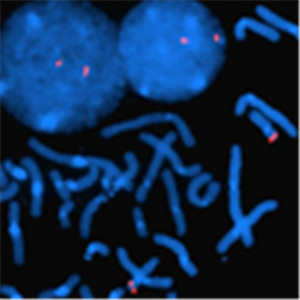
This probe localizes to the chromosome region 17p13 allowing it to detect chromosomal aberrations to the p53 gene. Somatic mutation of the p53 tumor suppressor gene is the most common genetic alteration seen in human cancers. Wild type p53 prevents genetic instability and participates in the apoptotic response to radiotherapy and chemotherapy. Mutations in p53 gene usually correlate with poor outcome and early recurrence in cancer.
Empire Genomics has developed a custom p53 probe which can be used to detect/confirm a rearrangement of the p53 gene. The probe comes labeled in orange, and generally ships within 24 hours. You can choose to customize the probe to meet your needs, which will ship right after production of the probe is complete (approximately 1 week).
Probe Details

| Gene: |
p53 (Orange 5-TAMRA dUTP) |
| Loci: |
17p13 |
| Test Kits: |
20 |
bio-equip.cn
The leading supplier of molecular diagnostics for customization and production of FISH probes, aGCH assays and upcoming new technologies to be used by Reference labs, Academic labs, Pharma, CRO’s
and Pharma /Biotech companies around the world.
Our companion Diagnostics will allow you to :
-Validate Bio-markers (signature identification and resistance).
-Identify and qualify the aberration at the Chromosomal level of specific population.
-Follow up the result of therapeutic treatment (monitoring, compound profiling, drug response, patient stratification and safety)
-Compare with the result of your current technology.
-Be translated into the initial phase of your diagnostics development toward new FDA filings (pre-clinical through clinical trial development).