Nano-Micro offers a series of hydrophobic interaction chromatography (HIC) media for high efficiency separation and purification of proteins and peptides. HIC is used to separate proteins on the basis of relative hydrophobicity. The unique feature of HIC is that proteins bind at high salt concentration and elute at low salt concentration. While in reversed phase column chromatography (RPC), desorption is often promoted by the addition of an increasing amount of organic solvent. Therefore gentle binding and elution condition of HIC allows a better retaining of the biologic activity of the target protein than RPC in which organic solvents are applied. The strength of the hydrophobic interaction is influenced strongly by the nature of the salt components in the mobile phase. The salt concentration needed depends on the protein hydrophobicity and solubility. HIC is particularly attractive for protein purification after ion exchange and affinity chromatography, where the sample with high salt concentration can often be applied directly to HIC. By combining HIC with ion exchange or affinity chromatography, the purification of biomolecules can be completed efficiently.


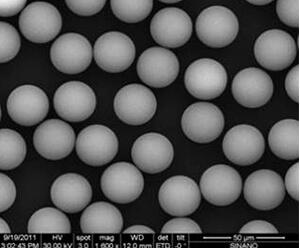
Highly uniform particle size
Uni®HIC are designed to deliver high resolution and high recovery for both lab and large-scale purification. Highly uniform particle sizes of 15, 30, or 80 μm with a narrow particle size distribution are available.
Superior chemical and pH stability

Comparison of column efficiency after treatment with acid and base
High Binding Capacity

Dynamic binding capacity (DBC) of Nano-Micro and competitor HIC resins
Products List

bio-equip.cn