The xGen® Pan-Cancer Panel v1.5 consists of 7816 individually synthesized and quality controlled xGen Lockdown® Probes that allow enrichment of 127 significantly mutated genes implicated across 12 tumor tissue types to enable deeper coverage during sequencing.
Benefits
- High uniformity across all targets
- Short list of driver mutations relevant to multiple cancer types
- Easily expanded by adding custom xGen® Lockdown® Probes
- Can be spiked into Nimblegen or Illumina Exome capture kits to enhance sensitivity of cancer exome sequencing
- Easy online ordering and next day shipping
Features
- 7816 xGen® Lockdown® Probes targeting 127 genes
- Individually synthesized and quality controlled probes
- Targets defined by whole genome and exome sequencing of >3000 tumors from 12 cancer types
- Validated with the xGen® Rapid Capture Protocol developed at IDT
Introduction
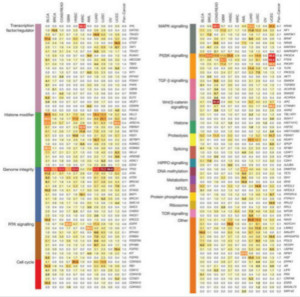
Every year, ~500 people in every 100,000 are diagnosed with cancer. Of these, 34% will not survive past five years. Cancer resulted in more than 500,000 deaths in 2013 alone [1]. Next generation sequencing has enabled the discovery and characterization of gene-specific mutations that have the potential to be tumorigenic; however, the technology also implicates a large number of genes that are not relevant to an individual’s cancerous state. A short list of mutated genes that are relevant to a multitude of cancer types, and that can be expanded to include additional cancer type–specific genes, would be invaluable in clinical and research applications. The Cancer Genome Atlas (TCGA) network performed a systematic analysis of more than 3000 tumors from 12 cancer types to investigate underlying mechanisms of cancer initiation and progression and have identified 127 significantly mutated genes (SMGs) across these tumor types [2].
IDT has developed the xGen® Pan-Cancer Panel, a hybrid capture panel that is based on the findings of the TCGA network, using xGen® Lockdown® Probes. Lockdown Probes are 120mer oligonucleotides bearing a 5′ biotin modification and manufactured using IDT Ultramer® synthesis technology.
References
- SEER Stat Fact Sheets: All Cancer Sites. National Cancer Institute. Surveillance, Epidemiology, and End Results Program. http://seer.cancer.gov/statfacts/html/all.html. (Accessed Feb 2014.)
- Kandoth C, McLellan MD, et al. (2013) Mutational landscape and significance across 12 major cancer types. Nature, 502:333–339.
Panel

FIGURE 1. SIGNIFICANTLY MUTATED GENES ACROSS 12 CANCER TYPES. 127 genes found to be significantly mutated in 12 cancer types are shown organized by gene pathway. The percentage of samples that contain the mutated form of the gene is shown for each of the cancer types. The highest percentage for each cancer type is shown in bold text. [Source: Kandoth, et al. (2013) Nature 502:333–339.]
bio-equip.cn