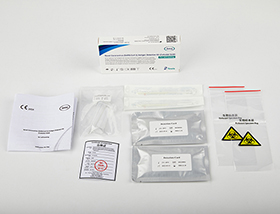
Intended Use
This kit is used to qualitatively detect the novel coronavirus (SARS-CoV-2) nucleocapsid protein antigen in human nasopharyngeal swab, oropharyngeal swab, nasal swab, and
saliva samples in vitro.
In the acute phase of infection, antigens are usually detectable in upper respiratory tract specimens. The rapid diagnosis of SARS-CoV-2 infection will help medical professionals treat patients and control the disease more effectively. The kit is designed for professional use only.
Product Description
CE&MHRA certificate self testing antigen rapid test kit
| Specification |
2tests/kit |
| Test Method |
Colloidal Gold |
| Power source |
Manual |
| Shelf life |
See manufacture date on label |
| Qualification |
EU CE, ISO13485:2016 |
| Function |
Home test |
| Service |
OEM service are avaliable |

Test Procedure
1. Before testing, equilibrate the extraction tube that contains sample extraction buffer to room temperature. Insert the extraction tube into the work station. Make sure that the tube is standing firmly and reaches the bottom of the work station.
2. Tear off the aluminum foil of the extraction tube. Insert the collected nasal swab sample into the extraction buffer.
3. Rotate the swab at least 6 times while pressing the swab tip against the bottom and side of the extraction tube.
4. Leave the swab in the extraction tube for 1 minute.
5. Squeeze the tube for several times with fingers from outside of the tube to completely immerse the swab.
6. Put the swab into a biohazard specimen bag. Cover the extraction tube with the dropper tightly. The extracted solution will be used as test sample.
7.Allow the detection card and test sample solution to equilibrate to room temperature. Take out the detection card from the package just before testing and place it on a clean and level surface.
8.Reverse the sample extraction tube, with the dropper downwards. Add 4 drops of test sample solution (by squeezing the tube) into the sample well.
9.Wait for the colored band(s) to appear. The results should be read in 15 minutes.
Do not interpret the results longer than 15 minutes. After observing and recording the results, put the detection card into the biohazard specimen bag and discard.
FAQ
1.Delivery date
A:leading time of sample order is about 3-5 wordays.
2.Shipment
A:We usually ship by DHL, UPS, FedEx. It usually takes 3-5 days to arrive.Airline and sea shipping is also optional.
3.If you accept OEM?
A:yes
4.Will you help me register the products in my country?
A:we would like offer technical documents and support to help you to register the products until the registration is finished.
5.Do you have MOQ?
Low MOQ, 1pc for sample checking is available
bio-equip.cn