Introduction
Dog flu is also caused by a specific virus of the influenza virus . But have quite different with human influenza virus and bird flu virus.
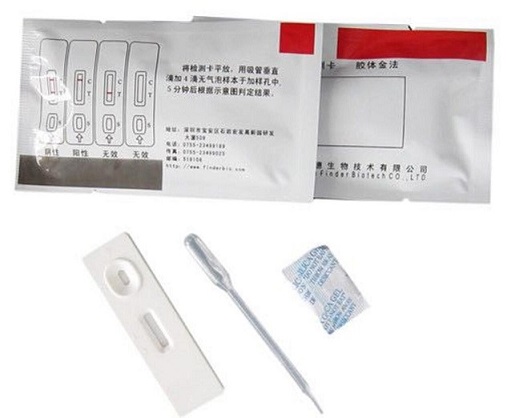
The Rapid CIV Ag Test Kit use chromatographic immunoassay for the qualitative detection of canine Flu virus antigen in dog`s nasal discharge. After adding sample, it moves along with labled CIV antibody. If there is CIV antigen, combine with antibody on T line showing wine red color. If no CV antigen, no color reaction.
Contents
|
1
|
Test Device
|
10 pcs
|
|
2
|
Dropper
|
10 pcs
|
|
3
|
Swab
|
10 pcs
|
|
4
|
Dilution Buffer
|
10 bottle
|
|
5
|
Instruction
|
1 insert
|
Procedure
1) Use swab to collect sample from dog`s nasal or pharyngealo Insert the swab into sample buffer ,Mix and stirto make it dissolved compleate.y.
- Remove the test device from the foil pouch, and place it on a flat and dry surface.Using the disposable dropper provided,Add 5 drops of sample into well marked “S” on the device.
- As the test begins to work, you will see purple color move across the result window in the center of the test device. If the migration has not appeared after 1 minute,you can press areas between " sample hole" and "T line" , help liquid release.
- Read the test results within 10 ~ 15 minutes, Invalid result after 15 minutes
Results
- Positive:Both on T Line and C line being seen wine-red color reaction.
- Negative:no color reaction on Test Line (T Line),only on Control Line(C Line) being seen wine-red color reaction.
- Void:No color reaction on C Line.
Package
10 tests/kit
Shelf Life
24 months (Lot No. printed on aluminum foil)
Storage
2-30 ℃
Notice
1. Aluminum foil bags damaged products can not be used. Must be used within 1 hour after open the package.
2.If the serum can not be tested immediately, store at 4℃ for short time (in 48hours), store at below -20℃ for long-tern storage.
3. The serum sample must be clarified and if any visible particles are present, centrifugation at 3,000 rpm for 20 minutes.
4. Adding too much sample will cause the false reaction, and overdosing dilution buffer can also lead to incorrect results.
5. Experiment is effective and quality control line (C line) no correlation. As long as the quality control line is clearly visible, it means the experiment is effective.
6. There is no potential bio-hazard for the antigen used in the test card, but all serum samples should be disposed of properly in accordance with the local pollutant treatment regulations.
7. Expired product can not be used.
bio-equip.cn