Home > News > Cherwell to Highlight How Expanding EM Portfolio Solves Annex 1 Challenges at Cell & Gene Therapy Conference
Cherwell to Highlight How Expanding EM Portfolio Solves Annex 1 Challenges at Cell & Gene Therapy Conference
New RMM system addition to product range means Cherwell can fully support EM programmes for Annex 1 compliant contamination control strategies
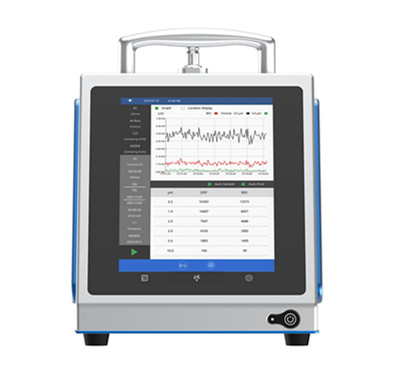

The new portable MicronView BioAerosol Monitoring System (BAMS) for RMM applications available from Cherwell in the UK & Ireland.
Bicester, UK, 8 June 2023: Cherwell, specialists in cleanroom microbiology solutions for the pharmaceutical, healthcare and related industries, is to highlight how its expanding environmental monitoring (EM) product portfolio can fully support the safe production of ATMPs in line with new EU GMP Annex 1 regulations. At the Cell & Gene Therapy Conference in London, 19-20th June, EM experts on Cherwell’s stand will advise on the best EM solutions to enable holistic, facility wide contamination control strategies (CCS) to manage risk in the manufacture of sterile medicinal products. Solutions include a new portable Rapid Microbiological Monitoring (RMM) system recently added to their range.
The Cell & Gene Therapy (CGT) conference will explore the latest industry approaches in the development of cancer and rare disease treatments, such as personalised cancer vaccines, stem-cell treatments for heart failure and haemophilia gene therapies. Presentations with real-world examples from key CGT companies will include discussion on advanced therapy medicinal product (ATMP) production, such as the GMP requirements for lentiviral vector manufacturing.
Revised in 2022 with an implementation deadline of 25th August 2023, Annex 1 is central to GMP guidelines governing the manufacture of sterile medicinal products, including CGT, within the UK and Europe. To meet Annex 1 requirements, manufacturers must have a clear rationale for assessing risk and preventing contamination of finished products by micro-organism, particulate or pyrogen. EM is key to this; notably a continuous monitoring approach in Grade A environments is now required.
Delegates to the conference will have the opportunity to see some of the latest microbiological EM solutions in Cherwell’s portfolio designed to cover all graded spaces (A – D). This will include the first truly portable RMM system. The newly introduced MicronView BioAerosol Monitoring System (BAMS) is a Biofluorescent Particle Counter (BFPC) that enables the rapid real-time, continuous monitoring of airborne microbes to support the new Annex 1 requirements, as well as early detection applications.
The BAMS early detection system reduces risk to patients as it enables focused investigation into potential contamination during real-time campaigns. It can lead to separation of a specific section of the manufactured batch and aid in the quick assessment of available data. This reduces the cost and waste of product if a real-time decision can be made, especially for a short shelf-life product that requires rapid release.
As a complementary tool to traditional culture-based methods, BAMS is an ISO certified particle detector and easily used in many critical locations to offer continuous monitoring, real-time feedback, and trending in aseptic environments, which are all detailed in the 2022 Annex 1 revision. Using laser induced fluorescence, the new particle detector can detect Active Fluorescent Units (AFU) and count viable microbes without need for culturing, staining or reagents. With the introduction of the BAMS RMM system, Cherwell now offers a complete environmental monitoring (EM) portfolio to meet all cleanroom microbiology needs.
“A stringent EM program during manufacture is critical to the safety and success of biological products such as cell & gene therapies for the targeting of undruggable diseases,” said Hamish Hogg, Microbiology Product Specialist, Cherwell. “For best practice EM, a combination of passive sampling and active air samplers, together with accessories to ensure precise data collection is required. The introduction of BAMS together with the rest of our range, means that we can consult with and offer our customers the solution that best fits their requirements.”
Alongside BAMS, also to be highlighted on Cherwell’s stand will be its EM portfolio of cleanroom microbiology solutions supporting both passive and active viable particle detection applications, plus microbial identification. This includes the Redipor® prepared media range, comprising agar plates, bottled media, broth bags and ampoules, vials and DIN bottles, and SAS microbial air samplers.
For more information about Cherwell Laboratories, please visit www.cherwell-labs.co.uk, follow @CherwellLabs on Twitter or follow us on LinkedIn.
This release is also available online at: https://www.cherwell-labs.co.uk/cherwell-labs-post/cherwell-to-highlight-how-expanding-em-portfolio-solves-annex-1-challenges-at-cell-gene-therapy-conference
ABOUT CHERWELL LABORATORIES: www.cherwell-labs.co.uk
Located in Bicester, central England, Cherwell Laboratories provides standard and bespoke cleanroom microbiology solutions that help customers effectively manage their controlled environments and processes. Established for 50 years, the company has built a strong reputation as a leading supplier of prepared microbiological media and environmental monitoring equipment, with considerable expertise within the pharmaceutical and related industries.
Cherwell manufactures its extensive range of Redipor® prepared media products in its ISO9001 registered facility, and has the flexibility to respond to varying customer requirements - from routine high volume down to low volume specialist applications. In-house engineering facilities support production and the development of bespoke solutions, as well as the supply, service and calibration of its environmental air monitoring equipment solutions.
Cherwell builds strong partnerships through professional, responsive and open communication, and by delivering high quality products, plus a flexible and reliable service with expert support, Cherwell ultimately helps its customers to reduce risk.
Related News
- Fluoropolymers Recently Diversified at Alfa Chemistry 4/18/2025
- Sabin Begins Marburg Vaccine Trial in U.S. 4/17/2025
- Titan Enterprises Powers Forward with Green Initiatives 4/16/2025
- Win a MINI 96 portable electronic pipette! 4/15/2025
- Eyestem’s Eyecyte-RPE™ Trial Shows Vision Rescue for Geographic Atrophy Pa 4/15/2025
- BOC Sciences Returns to Drug Discovery Chemistry 2025, Seeking Broad Partnership 3/28/2025
- INTEGRA Biosciences Details the Evolution and Future of Serological Pipet 3/28/2025
- Merck and Zebra Technologies Collaborate to Create Safety and Traceability Solut 3/28/2025
- Junshi Biosciences Announces Toripalimab’s Approval in Singapore 3/27/2025
- Alfa Chemistry Enhances Product Accessibility for Fluorescent Tracers wit 3/27/2025


