Home > News > EKF Showcases New Csolution for Point-of-care HbA1c Analyzers at Medica 2016
EKF Showcases New Csolution for Point-of-care HbA1c Analyzers at Medica 2016
Connectivity package for Quo-Lab™ and Quo-Test™ analyzers uses
POCT1-A2 communication protocol for diabetic HbA1c testing.
Cardiff, UK – 3rd November 2016 – EKF Diagnostics, the global in vitro diagnostics company, announces that it will be previewing a new point-of-care (POC) connectivity solution for its range of HbA1c analyzers at Medica 2016 in Düsseldorf from 14–17th November. In Hall 3 / C70, EKF will be showcasing the new connectivity package for Quo-Test™ and Quo-Lab™ HbA1c analyzers, enabling secure bi-directional communication between these POC analyzers and a multitude of central data management systems. Using the industry recognized POCT1-A2 communication protocol, EKF’s connectivity solution unlocks a host of new features aimed at improving security and quality control (QC) for diabetic HbA1c testing.
The new connectivity package includes a proprietary connector interface box, cables and a software upgrade. This enables EKF’s HbA1c analyzers to automatically transmit patient data to the majority of Lab Information Management Systems (LIMS) or Hospital Information Systems (HIS). Traceability and results recall speed are improved by use of patient ID and increased demographic data (such as family name and date of birth). These can now be recorded through either selecting from a pre-approved list or via the barcode scanner and keyboard.
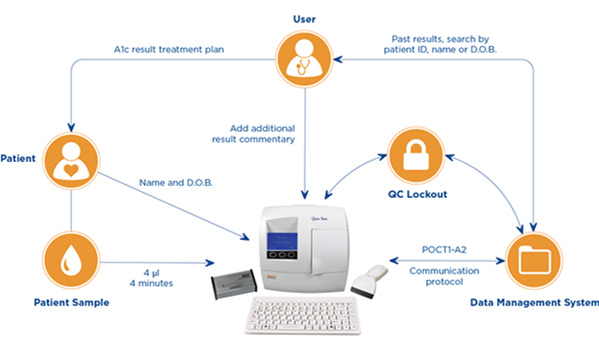
EKF’s Quo-Test™ and Quo-Lab™ now come with a connectivity package allowing
these point-of-care HbA1c analyzers to transmit patient data to the majority of LIMS and HIS.
Also ensuring the integrity of results generated at the POC, security and QC is enhanced on Quo-Lab and Quo-Test through user ID and QC lockout functions which are included in the connectivity package. By restricting access to trained users only, the lockout functions minimize the chances of user error and adhere to security protocols in many institutions. Furthermore, unauthorized users will be prevented from accessing patient information. Multiple user-defined QC lockout options are also available to POCT coordinators in order to enforce regular testing of QC materials and ensure compliance.
“HbA1c, or glycated haemoglobin, is well recognised as a reliable measure for glycemic control and managing patients with diabetes. As HbA1c levels reflect average circulating glucose concentration over a two to three month period, this means that it can offer greater clinical information than a single glucose measurement,” said Gavin Jones, Diabetes Product Manager, EKF Diagnostics. “Our Quo-Lab and Quo-Test analyzers deliver lab-quality results for HbA1c from 4µL of blood within 4 minutes, enabling the consulting clinician to give immediate feedback to a patient. In addition to ensuring the reliability of results, our new connectivity package also allows the clinician to add commentary to any test result, further enhancing the monitoring and management of diabetes in a point-of-care setting.”
On EKF’s Stand C70, Hall 3, at Medica there will be live demonstrations of the new connectivity package for Quo-Lab and Quo-Test analyzers. In addition, other products from EKF’s in vitro diagnostics range for both point-of-care and central laboratory settings will be available to discuss and view. These will include the Hemo Control, DiaSpect Tm, Biosen C-Line and Lactate Scout+.
For more information on EKF Diagnostics, please see www.ekfdiagnostics.com.
About EKF Diagnostics - www.ekfdiagnostics.com
EKF Diagnostics Holdings plc, which includes the EKF Diagnostics, Stanbio Laboratory and DiaSpect brands, specializes in the development, production and worldwide distribution of point-of-care analyzers for use in the detection and management of diabetes, anemia, lactate and kidney related diseases. EKF products are sold in more than 100 countries around the globe, through a network of specialist distributors.
Point-of-care diagnostics: EKF Diagnostics designs and manufactures world-class diagnostic devices, as well as distributing rapid test kits for infectious diseases and pregnancy. The EKF analyzer range is used widely in GP surgeries, pharmacies, blood banks, sports clinics, hospitals and laboratories for glucose, lactate, hemoglobin, hematocrit and HbA1c measurement.
Central laboratory: EKF, through its wholly owned subsidiary, Stanbio Laboratory (Boerne, Texas, USA), manufactures a comprehensive range of clinical chemistry reagents, as well as associated analyzers. In addition, EKF Life Sciences (Elkhart, Indiana, USA) manufactures enzymes used in reagent development and also provides contract fermentation facilities.
Related News
- Protheragen-ING Offers a Full Range of Nonsteroidal Anti-inflammatory APIs 2/21/2025
- Alfa Chemistry Unveils Cutting-Edge Functional Fibers: Nanofiber, Polymer Optica 2/19/2025
- New CEOs for Life Science and Healthcare Business Sectors and New Chief People O 2/19/2025
- Navigating FDA 510(k) Approval with Proregulations' Services 2/18/2025
- Nanoformulation Pilot Scale-up: CD Formulation’s Expertise in Customizing and Ma 2/17/2025
- JuliaHub Doubles Investment in Advanced Pharmaceutical Modeling Platform 2/14/2025
- Alfa Chemistry: Advancing Biotechnology with Renewed Oligonucleotide Product Lin 2/14/2025
- Tillotts Pharma AG Celebrates 15 Years as an Integral Part of the Japanese Zeria 2/14/2025
- Leading Expert Discusses Women in Science 2/7/2025
- Thermo Fisher Scientific Launches International CorEvitas Clinical Registry in A 2/6/2025


