OGT to launch cancer sequencing panel at ACGS
Accurate solid tumour profiling for clinical and translational research
Oxford, UK – 29 April 2014. Oxford Gene Technology (OGT), The Molecular Genetics Company, will launch its new SureSeq™ Solid Tumour Panel at the Association for Clinical Genetic Science (ACGS) meeting at Birmingham UK, on the 29-30th April. Fully validated on formalin-fixed, paraffin-embedded (FFPE) samples, the new 60-gene next generation sequencing (NGS) hybridisation-based enrichment panel offers researchers accurate and reliable solid tumour profiling for both known and novel variants.
The content of the panel has been defined by recognised cancer experts, covering key genes for a range of cancer types including breast, prostate, ovarian, lung and colorectal. All exons of these genes are fully covered, including mutation hotspots, enabling both detection and discovery of known and novel variants respectively.
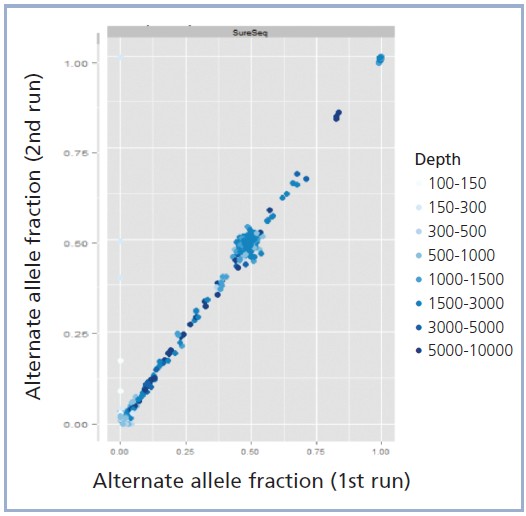
The hybridisation-based SureSeq Solid Tumour Panel minimises PCR bias and duplications commonly associated with alternative enrichment methodologies, enabling greater run-to-run consistency. This is particularly important in situations where there is limited sample or where the ability to detect minor allele frequencies is required, such as in heterogeneous tumour samples. Such sample types require a highly uniform and sensitive enrichment and OGT’s expert bait design ensures this by providing efficient and improved uniformity of coverage of the targeted regions, enabling all variants to be called with maximum confidence.
Providing easy access to meaningful data, the SureSeq Solid Tumour Panel comes with OGT’s unique Variant Analysis Report, equipping researchers with the freedom to explore and retrospectively interrogate data with additional or new selection criteria, without the need for additional in-house bioinformatics resource. Using the report, data can be easily filtered by numerous parameters, including gene, depth of coverage, somatic variants and predicted effect on the protein. In addition, all variants are fully annotated with links to various databases.
Delegates of the ACGS meeting can find out more about the new panel in a talk by Dr Matthew Smith (West Midlands Regional Genetics Laboratory) titled “Developing a 60 gene somatic cancer capture panel for consolidated tumour profiling” at 12.20pm on 30th April 2014.
To find out more, please visit the website.
For further information, please contact:
Oxford Gene Technology, Begbroke Science Park, Begbroke Hill, Woodstock Road, Begbroke, Oxfordshire, OX5 1PF, U.K.
T: +44 (0) 1865 856826 ; F: +44 (0) 1865 848684
E: contact@ogt.com ; W: www.ogt.com ; Twitter: @OxfordGeneTech
About Oxford Gene Technology
Oxford Gene Technology (OGT) provides world-class genetics research solutions to leading clinical and academic research institutions. Founded by Professor Sir Edwin Southern, and with customers in over 60 countries worldwide, OGT has a strong reputation and increasing share in the large and growing genomic medicine market. The Company’s Cytocell®, CytoSure™ and Genefficiency™ range of fluorescence in situ hybridisation (FISH), microarray and next generation sequencing (NGS) products and services deliver high-quality genetic analysis, enabling accurate identification and confirmation of the causative variation underlying genetic disease.
For more information on the Company, please visit our website at www.ogt.com
CytoSure™ and Genefficiency™ NGS browser/report: For Research Use Only; Not for Use in Diagnostic Procedures
CytoSure: This product is provided under an agreement between Agilent Technologies, Inc., and OGT. The manufacture, use, sale or import of this product may be subject to one or more of U.S. patents, pending applications, and corresponding international equivalents, owned by Agilent Technologies, Inc. The purchaser has the non-transferable right to use and consume the product for RESEARCH USE ONLY AND NOT for DIAGNOSTICS PROCEDURES. It is not intended for use, and should not be used, for the diagnosis, prevention, monitoring, treatment or alleviation of any disease or condition, or for the investigation of any physiological process, in any identifiable human, or for any other medical purpose.
- 4th Edition Drug Safety Symposium 2025 4/17/2025
- 3rd Annual Pharma Impurity Conclave 2025 4/17/2025
- 46th China (Beijing) International Medical Devices Exhibition 2025 4/17/2025
- analytica Vietnam 2025 Concludes with Record-Breaking Attendance and Exhibitor P 4/14/2025
- The Materials Research Summit 2025 4/14/2025
- analytica Vietnam 2025 Opens Tomorrow: The Leading Laboratory Exhibition Returns 4/1/2025
- MEDICAL JAPAN 2025 OSAKA Concludes with Strong Industry Attendance and New Busin 3/28/2025
- Pharma Partnering US Summit 2025 3/20/2025
- analytica Lab India 2025 3/13/2025
- Post Report: 8th Annual Pharma Project and Portfolio Management 2025 3/7/2025


