Evaluation Success for EKF’s POCT HbA1c Analyzers
Quo-Test and Quo-Lab analyzers meet set requirements for determination of HbA1c
|
Cardiff, UK – 22nd October 2013 – EKF Diagnostics, the global diagnostics business, announces the publication of a new white paper detailing the successful external evaluation of its Quo-Test and Quo-Lab glycated hemoglobin (HbA1c) analyzers - ‘Evaluation of four POCT-Systems for the measurement of HbA1c’. The aim of the study by scientists at IMCARMED GmbH was to compare four point-of-care (POCT) methods with established laboratory techniques for the determination of HbA1c, an internationally recognized biomarker of blood glucose levels for diabetes management. Bringing testing for this biomarker into a POC setting enables real-time monitoring and management of diabetes. Treatment programs utilizing POC HbA1c analysis are able to minimise the number of clinical consultations required and offer a better standard of clinical care to their patients. |
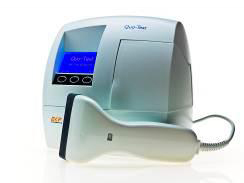 EKF’s Quo-Test HbA1c analyzer is easy-to-use and highly accurate in a POCT setting |
The accuracy trial of the four POCT methods was performed with 100 different EDTA blood samples over four days and the results were then compared with those gathered using laboratory-based HPLC and immunological methodologies. Intra-run precision was also examined and all four POCT systems demonstrated good repeatability.
The study concluded that all four POCT systems met the requirements of HbA1c determination in practice and those set by the German Medical Association. EKF’s Quo-Test was noted as being particularly easy-to-use and therefore ideal for use in a POCT setting by non-laboratory trained users. Additionally, with regard to accuracy Quo-Test was found to have the best agreement with both laboratory methods.
The new white paper ‘Evaluation of four POCT-Systems for the measurement of HbA1c’by Dr Andreas Müller (IMCARMED GmbH) is published online at: www.ekfdiagnostics.com/HbA1c_Analysers_115.aspx.
For more information on EKF Diagnostics, please visit www.ekfdiagnostics.com.
About EKF Diagnosticswww.ekfdiagnostics.com
EKF Diagnostics Holdings plc specializes in the development, production and worldwide distribution of point-of-care blood analyzers for use in the detection and management of diabetes, anemia, lactate and kidney related diseases. Its new Molecular division focuses on molecular and companion diagnostics.
Point-of-care diagnostics: EKF Diagnostics’ expertise covers the entire in vitro diagnostics chain, from fermentation and enzyme production, to liquid reagent manufacture, design and building of world-class diagnostic devices, and distribution of rapid test kits for infectious diseases and pregnancy. The EKF analyzer range is used widely in GP surgeries, pharmacies, blood banks, sports clinics, hospitals and laboratories for glucose, lactate, hemoglobin, hematocrit and HbA1c measurement.
Companion Diagnostics: In March 2013 EKF set up a new division to focus on molecular and companion diagnostics - EKF Molecular Diagnostics develops technologies for cancer gene detection. Through its acquisition of UK-based 360 Genomics and by offering innovative products with the potential to change current DNA extraction and detection practices, EKF is addressing the fast growing companion diagnostics market.
EKF Diagnostics products are sold in more than 100 countries around the globe. EKF Diagnostics’ strengths lie in its multi-national research and manufacturing facilities, teams of experienced analysts and engineers in Germany, Ireland, USA and the UK, and a board led by some of world’s foremost authorities in medical diagnostics.
EKF Diagnostics Holdings plc
Martyn Lewis
t: +44 (0)29 20 710570
e: martynlewis@ekfdiagnostics.com
w: www.ekfdiagnostics.com
- High Specification Optical Flats for Custom Fizeau Interferometer 12/18/2025
- Merck Launches ChemiSphere® App to Simplify Digital Data Access and Boost L 12/17/2025
- Breathing New Life into Older High-speed Cameras 12/16/2025
- Cold Chain Technologies Expands Industry-Leading Reusable Portfolio 12/11/2025
- Sabin Vaccine Institute’s Investigational Marburg Vaccine Delivered to Ethiopia 12/9/2025
- Cutting-edge Product for Stem Cell Research and Human Embryo Modelling 12/4/2025
- Thermo Fisher Scientific Launches Industry-First, Multi-Parameter Molecular Assa 11/20/2025
- Biotech Fluidics: Solvent Recyclers Improve HPLC System Sustainability 11/20/2025
- Titan Enterprises’ Beverage Flow Meters: Turbine vs. Ultrasonic – Which is Right 11/19/2025
- Gentle and Rapid Detachment of Even Delicate Adherent Cells 11/18/2025


