Home > News > 2019 Enmore BIO Conference
2019 Enmore BIO Conference
March 1st-3rd Nanjing, China
Organizer: Enmore healthcare
Here you can get close to the decision makers from China local giant CMO and bio pharma companies.
Since very beginning EBC has been the right place to get in touch with China Biotech industries. EBC brings a wide spectrum of life science and application areas including: Cell therapy, Antibody drugs, Diagnostics, Bioprocessing and Venture Investment.
We are honored to invite you to take part in 2019 ENMORE BIO CONFERENE(2019 EBC), Nanjing, China, which is the largest BIO Conference in China, and attracts 2500+ BIO tech KOL who come together of intensive networking to discover new opportunities and promising partnerships.
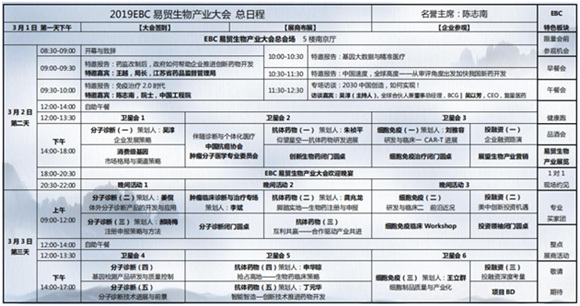
Co-Host (Antibody drugs)

Always focus on high quality drugs to improve patients’ life, for a better life.
Co-planer
Zhenping Zhu, R&D Director

Dr. Zhu has been engaged in the research and development of therapeutic antibodies for more than 30 years, and has more than 25 years of experience in the research and development and management of internationally renowned biological pharmaceutical enterprises. He has participated in and led the development of a number of therapeutic human anti-tumor antibodies, 4 of which have been approved by FDA and EMA in the United States and more than 15 have entered phase I to III clinical trials

Focusing on precision cancer medicine, we have been adhering to the core values of "patient-centered", developing the business model of integration of tumor diagnosis and treatment, and becoming a practitioner of integration of tumor diagnosis and treatment in China.
Co-planner
Zhaolong Gong, CEO

Dr. Gong g served as the new drug examiner of FDA from 1998 to 2008. After returning to China in 2008, he successively served as chief technical officer of Joinn-lab new drug, CEO of laibo pharmaceutical and vice President of Beigene new drug development and drug administration. From the FDA in recent 20 years, CRO and different angles, such as participating in new drug research and development of the enterprise, new drug research and development in the new global development strategy planning, non-clinical and clinical trial design, GLP and GCP regulations and new drug project selection and into medicinal properties evaluation, and coordination to promote the clinical experiments and clinical trials, and for the advance of the project risk assessment in the aspects such as accumulated rich experience. Currently, he is the editorial board member of new drug journal of China, the editorial board member of pharmaceutical progress journal, AAALAC council member, and the director of freehand brushwork club.
Co-Host (Cell Therapy)

I-Mab is an innovative biopharmaceutical company with an exclusive focus on biologics with first-in-class.
Co-planner
Huaqiong Shen, R&D, Clinical Director

Dr. Shen served as a global clinical research and development executive of Eli Lilly, Wyeth and Pfizer in the United States for more than 10 years. After returning to China, he joined Hengrui pharmaceutical and helped establish a clinical team of innovative drugs. He successfully developed clinical trials in Australia and the United States. Joined Johnson & Johnson in 2015, served as vice President and general manager of China development center, responsible for the registration trial in China and innovation projects in Asia.

Fosun Kite strives for the development and industrialization standardization of tumor immune cell therapy technology in China.
Co-planner
Liqun Wang,CEO

Dr Wang has rich research and development technology and management experience in the field of bio-pharmaceuticals in the United States and China. He has held key management positions in P&G, Bristol-Myers Squibb, AstraZeneca and GSK's R&D centers in China for nearly 20 years.

Adhering to the concept of curing and saving lives, we provide cutting-edge precision treatment programs for cancer patients.
Co-planner
Yarong Liu, R&D Director

Dr. Liu is an expert of "thousand talents project" in Shanghai and a doctor and postdoctoral fellow of university of southern California. She has been engaged in the research of three-generation CART in Amgen Company in the United States, adenovirus, lentivirus and other viral vectors for targeted gene therapy and for more than 10 years.
Co-Host (Molecular Diagnostics)
Co-Host (Molecular Diagnostics)
Chun Wu,CEO,BCG

Years of deep cultivation of health care industry, both management and consulting experience. Wu leads BCG's greater China healthcare business, leading the team to serve many domestic and foreign pharmaceutical companies, device companies, medical and insurance institutions. At the same time, she is committed to the system construction and application development of medical big data.

Independent third-party medical diagnostic service institution providing "integrated medical diagnostic services". Took the lead in listing in July 2011, achieving "zero breakthrough" in listing independent medical laboratories in China.
Co-planner
Tang Jiang,Senior Strategy & Technical Manager

Tang Jiang, researcher and doctoral supervisor. He was an associate researcher at Harvard medical school and a researcher at MD Anderson cancer center, university of Texas. SYSU medical college, chairman of the inspection department, the ministry of education medical technology teaching steering committee of the deputy chairman

Leading compliance consulting company in the field of medical devices in China. It has a new service category and service mode supported by ISO9001 quality system and information management system certified by SGS.
Co-planner
Xiaomei Hao,VP&Co-founder
 .
. Xiaomei Hao is a special expert of the senior research institute of the state administration of food and drug administration. National bureau of medical device technology review center subject laws and regulations and management support consultants; More than 10 years of compliance and operation management service experience in medical device field. With 15 years of experience in large enterprise management and compliance management, I am proficient in laws and regulations in the field of medical devices.
Co-Host (Precise Medical)
Professional committee of oncology molecular medicine, Chinese anticancer association

An innovation-driven biopharmaceutical company, which aims to develop and provide safe, effective, reliable and affordable clinical drugs in urgent need of biotechnology
Co-planner
Xinhua Zhou,CEO

Dr. Zhou has led the process development of biotechnology drugs and monoclonal antibodies in EntreMed and HGS successively, and served as the scientific director of process development of Amgen, the largest biological pharmaceutical company in the world. In October 2008, he returned to China and became CEO of Genor bio-pharmaceutical co., LTD., helping the development of bio-pharmaceutical industry in China
Co-Host (Workshop)
Co-Host

Co-planner
Tong Guo,Vice president of Great China Business Development

Dr. Guo is currently vice President of greater China business development at Aquun weir research and development, executive director of biostatistics for Asia Pacific and Africa at Aquun Wyatt, and holds a master's and doctor's degrees in biostatistics from McGill University, Canada. With nearly 20 years of experience in new drug research and development in large international pharmaceutical companies. Presided over the statistical design and analysis of several global multi-center clinical trials.
Contact:
Brandon Huang-Key account Manager
T: +86-21-51552421
W: http://ebc.enmorebiz.com
Related News
- 11th Microbiome R&D and Business Collaboration Congress and 2nd Skin Health & Mi 12/12/2025
- 4th Cell & Gene Therapy Innovation & Access Congress: Asia 12/12/2025
- Next Generation Genomics Congress: Asia 12/12/2025
- 3rd Edition London Biotechnology Show 12/10/2025
- 14th Alzheimer’s & Parkinson’s Drug Development Summit 12/3/2025
- Innovation and Partnership Take Centre Stage at the 93rd Edition of API China in 11/21/2025
- 16th Edition R&D Controlling and Performance Management 11/20/2025
- 9th Annual Pharma Project & Portfolio Management 2026 11/6/2025
- 5th Edition Drug Safety Symposium 2026 - Middle East Chapter 11/6/2025
- Oligonucleotide-Based Therapeutics Summit 10/22/2025


